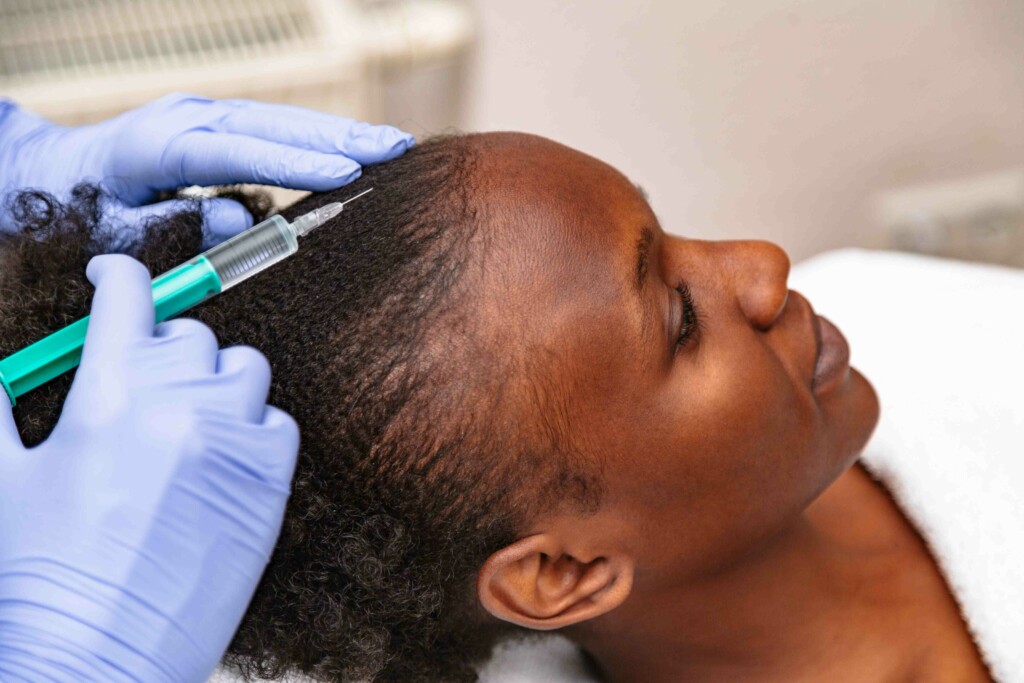
In the last few decades, topical minoxidil – an FDA-approved hair loss treatment for pattern hair loss (androgenetic alopecia) – has been used off-label to help people with cancer treatment-induced hair loss. Although this approach has been relatively successful, studies are now looking to explore the use of a different formulation: low-dose oral minoxidil. This alternative hair loss medication is even being explored as a treatment for children and adolescents with persistent alopecia following their cancer treatments. (1,2)
How Do Cancer Treatments Cause Hair Loss?
Chemotherapy, radiation therapy, and other anti-cancer therapeutics (like endocrine therapy) are known to have the potential to cause hair loss. Many forms of chemotherapy and radiotherapy often cause sudden, obvious alopecia – known as anagen effluvium – which causes hairs to break as soon as they reach the scalp’s surface. However, chemotherapy can also cause telogen effluvium, a form of alopecia that appears as diffuse hair shedding. Hormonal anti-cancer therapeutics like endocrine therapy are more likely to cause gradual, progressive hair thinning – essentially a prolonged form of telogen effluvium. (3,4)
Chemotherapy is a type of cancer treatment that works by targeting and killing rapidly dividing cancer cells. However, this medical treatment can also affect healthy cells in the body. Chemotherapy drugs kill cells that divide rapidly, including the cells in and around hair follicles. This is why many cancer patients who undergo chemotherapy experience hair thinning and shedding – and in some cases, chemotherapy-induced alopecia. (3)
When chemotherapy drugs are injected into the body, they interfere with normal cell division, which can affect the hair growth cycle. Chemotherapy drugs can also damage hair follicle cells, causing them to stop growing and eventually causing alopecia. The extent and duration of hair loss vary from person to person, depending on factors like the specific chemotherapy drugs used, the dose and duration of treatment, and the treatment regimen. (3)
Similarly, radiation therapy uses high-energy particles or waves to kill cancer cells. It works by damaging the DNA inside cancer cells, making it difficult for them to divide and grow. This common cancer treatment can also cause hair loss if radiation therapy is applied to areas near the scalp, such as the head or face. (3)
You should know that not all cancer treatments cause hair loss and not everyone who undergoes chemotherapy will experience alopecia. The likelihood of hair loss depends on the specific drugs used, the dose, and the duration of chemotherapy treatment, as well as individual factors like each patient’s age and genetics. (3)
How Does Minoxidil Help Hair Loss?
Minoxidil is a medication that works by dilating blood vessels. The oral version of this drug is currently used for exactly that purpose: it’s FDA-approved to reduce blood pressure. Topical minoxidil is an FDA-approved hair loss treatment that seems to work in the same way – but specifically increases blood flow to the scalp. This results in more blood, oxygen, and nutrients reaching hair follicles, which promotes hair growth and helps reverse hair loss.
Topical vs. Oral Minoxidil
While both topical and oral minoxidil are FDA-approved, only topical minoxidil is approved as a hair loss treatment. Topical minoxidil, which is made as a liquid or a foam in strengths of 2% and 5%, is specifically approved for pattern hair loss (also known as androgenetic alopecia). Many people are familiar with it by its original brand name, Rogaine. (2)
Oral minoxidil, on the other hand, is typically referred to by the brand name Loniten. Loniten tablets are meant to be taken in doses ranging from 2.5 milligrams to 10 milligrams daily to reduce high blood pressure. (5) At these doses, oral minoxidil may help improve hair regrowth. However, it’s usually not used for that purpose as it can cause hair growth in unwanted areas and other side effects. (2)
Both oral and topical minoxidil are being explored as potential treatments for persistent radiation therapy and chemotherapy-induced hair loss. While topical minoxidil is usually used off-label in the same concentrations and formulations, oral minoxidil for hair loss is used in lower doses that can range from 0.25 milligrams to 5 milligrams per day. (2,6,10-12)
Topical Minoxidil for Chemotherapy-Induced Alopecia
Topical minoxidil has been studied as a treatment for chemotherapy-induced hair loss since the 1990s. The first study, in 1991, assessed just six people receiving chemotherapy. It reported no benefit in the prevention of hair loss after they used 2% minoxidil solution twice a day. (7) In 1996, a study of a small group of women who used a 2% minoxidil solution in doses of 1 milliliter twice a day, both during chemotherapy and for 4 months after chemotherapy, found that topical minoxidil decreased the duration of chemotherapy-induced alopecia. (8)
“Some patients are so concerned about hair loss with chemotherapy that they may refuse potentially lifesaving treatments. Hair loss is a marker of illness and a heavy burden; anything that offers hope is of interest. This treatment offers advantages over some used in the past in that it does not block chemotherapy from reaching the scalp, and may even enhance drug delivery by vasodilatation.” – NH Shear, reviewing Duvic M et al. J Am Acad Dermatol 1996 (9)
While these results may not seem particularly impressive, at the time, topical minoxidil had only just been approved by the US Food and Drug Administration for the treatment of androgenetic alopecia. More than that – it was the first hair loss treatment to have ever been approved – so to find that it was safe for patients receiving cancer treatments was monumental. These days, topical minoxidil is regularly used off-label to diminish hair loss and improve hair regrowth in people receiving cancer treatments. (2)
Oral Minoxidil for Chemotherapy-Induced Alopecia
Studies using oral minoxidil as a treatment for chemotherapy-induced alopecia are limited. However, between 2015 and 2022, several case studies were released highlighting oral minoxidil’s effectiveness. All of these case studies featured patients who had persistent or permanent chemotherapy-induced alopecia and whose treatment resulted in substantial or complete hair regrowth. The daily dosing of the oral minoxidil used in these studies ranged between 1 milligram and 5 milligrams. (10-12)
In 2022, a retrospective study was conducted exploring the effectiveness of oral minoxidil in female breast cancer patients. A total of 216 women with persistent alopecia were treated with 1.25 milligrams of oral minoxidil each day. Each patient received either chemotherapy, chemotherapy followed by endocrine therapy, or endocrine monotherapy. Low-dose oral minoxidil was able to improve their frontal and occipital hair density without causing any serious side effects. This study remains the largest one to date on the use of oral minoxidil for cancer patients. (6)
Given these promising results, a new Phase II clinical trial has recently been launched to evaluate the safety and efficacy of oral minoxidil in promoting hair regrowth in young cancer survivors. The trial aims to recruit 60 participants between the ages of 6 to 18 who have experienced persistent hair loss due to either chemotherapy or radiation therapy. (1)
The participants will be randomized to receive either oral minoxidil for 8 months or a placebo for 4 months, followed by oral minoxidil for 4 months. Given the variation in ages (and consequently, weights), dosing is weight-dependent (0.01 milligrams per kilogram per day for individuals under 40 kilograms and 0.02 milligrams per kilogram per day for individuals at or over 40 kilograms). The trial, which is expected to be completed by 2026, will provide valuable information about the safety and efficacy of oral minoxidil in pediatric cancer survivors. (1)
Combined Topical and Oral Minoxidil for Chemotherapy-Induced Alopecia
In 2022, another retrospective study was released on the use of oral minoxidil on breast cancer patients receiving chemotherapy or chemotherapy treatment followed by endocrine therapy. In this study, however, 100 women were given either a 5% topical minoxidil solution (meant to be applied once a day) or a 5% topical minoxidil solution and between 1.25 milligrams to 5 milligrams of oral minoxidil. The oral minoxidil-topical minoxidil group saw better improvements in hair density, but they also experienced more side effects, such as unwanted hair growth. Notably, one person was not able to tolerate the oral minoxidil as it caused swelling around their eyes. (13)
Which Is Better: Oral or Topical Minoxidil?
Topical minoxidil has been shown to be effective in promoting hair regrowth in some individuals during or following their cancer treatments. It’s also already approved as an over-the-counter pattern hair loss treatment for both men and women, which means that it’s considered to be safe, effective, and should be easy for most people to acquire.
Low-dose oral minoxidil has previously been shown to be more effective than topical minoxidil. (14) But this hasn’t been proven in cancer patients or after chemotherapy treatment. It’s also unclear what dosing is ideal for this prescription-only medication. More importantly, while generally considered to be safe, low-dose oral minoxidil has a higher risk of side effects that should be discussed with the physician prescribing this medication.
More research is needed to evaluate the potential benefits and risks of using oral minoxidil in cancer survivors. However, if topical minoxidil isn’t working for you, talk to your doctor about oral minoxidil. They should be able to determine if it’s a suitable medication for you and create a dosing plan that fits your needs.
References
- Memorial Sloan Kettering Cancer Center (2023). A Study of Oral Minoxidil to Treat Hair Loss in Children, Teens, and Young Adults Who Are Cancer Survivors(Clinical Trial Registration No. NCT05778825). clinicaltrials.gov
- Badri, T., Nessel, T. A., & Kumar, D. (2021). Minoxidil. In StatPearls [Internet]. StatPearls Publishing.
- Yeager, C. E., & Olsen, E. A. (2011). Treatment of chemotherapy‐induced alopecia. Dermatologic therapy, 24(4), 432-442.
- Side Effects: Hair Loss (2023) Breastcancer.org
- Loniten Minoxidil tablets, USP Warnings. Food and Drug Administration. (n.d.).
- Kuo, A.M.S., Reingold, R.E., Ketosugbo, K., Pan, A., Dusza, S.W., Kraehenbuehl, L., Gajria, D., Lake, D.E., Bromberg, J., Goldfarb, S.B. and Traina, T.A., 2022. Oral minoxidil for the treatment of late alopecia in cancer survivors.
- Granai, C. O., Frederickson, H., Gajewski, W., Goodman, A., Goldstein, A., & Baden, H. (1991). The use of minoxidil to attempt to prevent alopecia during chemotherapy for gynecologic malignancies. European journal of gynaecological oncology, 12(2), 129-13
- Duvic, M., Lemak, N. A., Valero, V., Hymes, S. R., Farmer, K. L., Hortobagyi, G. N., … & Compton, L. D. (1996). A randomized trial of minoxidil in chemotherapy-induced alopecia. Journal of the American Academy of Dermatology, 35(1), 74-78.
- Shear N.H. (1996) Topical Minoxidil for Chemotherapy-Induced Hair Loss. Dermatology
- Lyakhovitsky, A., Segal, O., Maly, A., Zlotogorski, A., & Barzilai, A. (2022). Permanent chemotherapy-induced alopecia after hematopoietic stem cell transplantation treated with low-dose oral minoxidil. JAAD Case Reports, 22, 64-67.
- Martora, F., Vastarella, M., Fattore, D., Patri, A., Fabbrocini, G., & Cantelli, M. (2022). Oral Minoxidil for Chemotherapy-Induced Alopecia. Skin Appendage Disorders, 8(6), 508-510.
- Yang, X., & Thai, K. E. (2016). Treatment of permanent chemotherapy‐induced alopecia with low dose oral minoxidil. Australasian Journal of Dermatology, 57(4), e130-e132.
- Kang, J., Lee, J. W., & Kwon, O. (2023). Efficacy of low-dose oral minoxidil in the management of anticancer therapy-induced alopecia in patients with breast cancer: A retrospective cohort study. Journal of the American Academy of Dermatology, 88(5), 1170
- Ramos, P. M., Sinclair, R. D., Kasprzak, M., & Miot, H. A. (2020). Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: a randomized clinical trial. Journal of the American Academy of Dermatology, 82(1)
Last updated December 2023